Withdrawal Letter Ibandronic Acid Hexal

 Notifying A Change Of Marketing Status European Medicines
Notifying A Change Of Marketing Status European Medicines
Janacti Inn Sitagliptin Pioglitazone
 Post Authorisation Measures Questions And Answers
Post Authorisation Measures Questions And Answers
 Cover Letter Template For Notification Of Withdrawn Products By
Cover Letter Template For Notification Of Withdrawn Products By
 Applying For Orphan Designation European Medicines Agency
Applying For Orphan Designation European Medicines Agency
 What We Publish On Medicines And When European Medicines
What We Publish On Medicines And When European Medicines
 Roche Withdraws Ema Application For Tecentriq Avastin
Roche Withdraws Ema Application For Tecentriq Avastin
 Worksharing Questions And Answers European Medicines Agency
Worksharing Questions And Answers European Medicines Agency
 Ema S Prac Calls For Fenspiride Meds Withdrawal And Xeljanz
Ema S Prac Calls For Fenspiride Meds Withdrawal And Xeljanz
 Periodic Safety Update Reports Psurs European Medicines
Periodic Safety Update Reports Psurs European Medicines
 A New Age Of Transparency Do We Fully Understand The
A New Age Of Transparency Do We Fully Understand The
 Pre Authorisation Guidance European Medicines Agency
Pre Authorisation Guidance European Medicines Agency
:max_bytes(150000):strip_icc()/2062559v1-5ba4f9604cedfd0025e0e729.png) Sample Letters Withdrawing A Job Application
Sample Letters Withdrawing A Job Application
 Drugs And Devices Comparison Of European And U S Approval
Drugs And Devices Comparison Of European And U S Approval
 Pre Authorisation Guidance European Medicines Agency
Pre Authorisation Guidance European Medicines Agency
 Gmp Oversight Of Medicines Manufacturers In The European Union
Gmp Oversight Of Medicines Manufacturers In The European Union
 European Medicines Agency Policy On Publication Of Clinical
European Medicines Agency Policy On Publication Of Clinical
 Regulatory Review Of Novel Therapeutics Comparison Of
Regulatory Review Of Novel Therapeutics Comparison Of
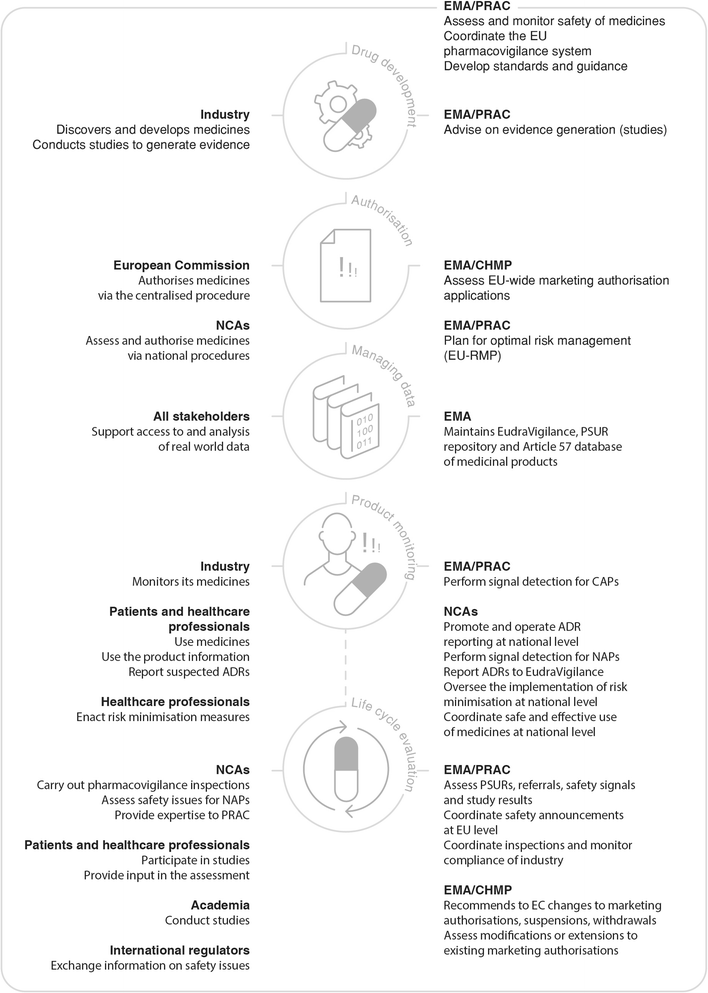 Promoting And Protecting Public Health How The European
Promoting And Protecting Public Health How The European
 Ema Guidance Based On Hard Brexit Impact For Medical Devices
Ema Guidance Based On Hard Brexit Impact For Medical Devices
Data Requirements To Demonstrate Biosimilarity In The Eu
 Ema Acknowledges Persistent Sexual Dysfunction After Ssris
Ema Acknowledges Persistent Sexual Dysfunction After Ssris
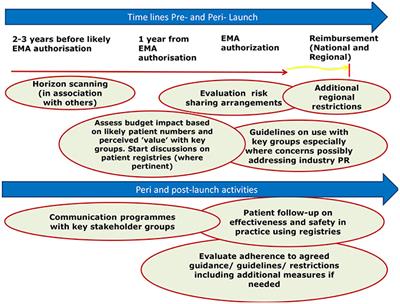 Frontiers Adaptive Pathways Possible Next Steps For
Frontiers Adaptive Pathways Possible Next Steps For
 The Food And Drug Administration Reports Provided More Data
The Food And Drug Administration Reports Provided More Data
 Glenis Willmott On Twitter More Delays To Eu Clinical
Glenis Willmott On Twitter More Delays To Eu Clinical
 One Of Two Pfizer Adalimumab Biosimilar Applications
One Of Two Pfizer Adalimumab Biosimilar Applications
 Brexit Medicines Medical Devices And Substances Of Human
Brexit Medicines Medical Devices And Substances Of Human
 Britain Loses Medicines Contracts As Eu Body Anticipates
Britain Loses Medicines Contracts As Eu Body Anticipates
 Periodic Safety Update Reports Psurs European Medicines
Periodic Safety Update Reports Psurs European Medicines
 Gospel And Culture From The Didache To Origen Richard
Gospel And Culture From The Didache To Origen Richard
 The European Medicines Agency Ema Logo Cyprus Mail
The European Medicines Agency Ema Logo Cyprus Mail
 Frustrated By Brexit Too High A Hurdle To Overcome For The
Frustrated By Brexit Too High A Hurdle To Overcome For The
 Ema Officially Welcomed To New Premises In Amsterdam I
Ema Officially Welcomed To New Premises In Amsterdam I
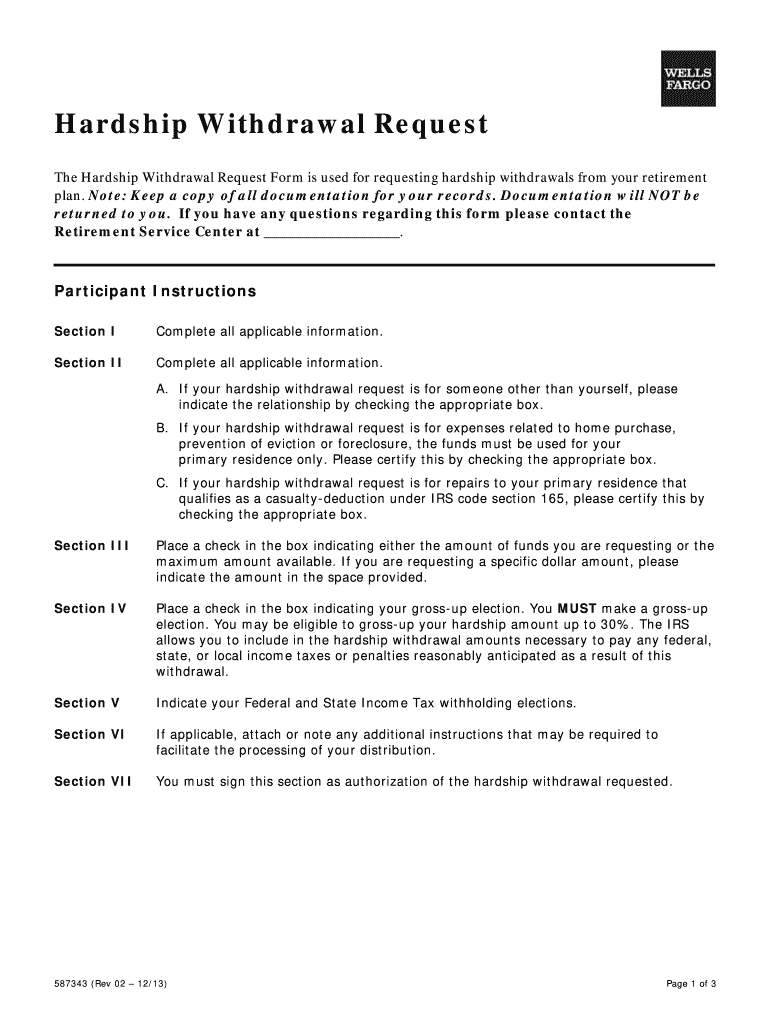 2013 2019 Form Wells Fargo 587343 Fill Online Printable
2013 2019 Form Wells Fargo 587343 Fill Online Printable
 Vintage Frame Effect Over Woman Finger Pressing The Z Letter
Vintage Frame Effect Over Woman Finger Pressing The Z Letter
Ema A Partner In Defence Of The Environment Trinidad Guardian
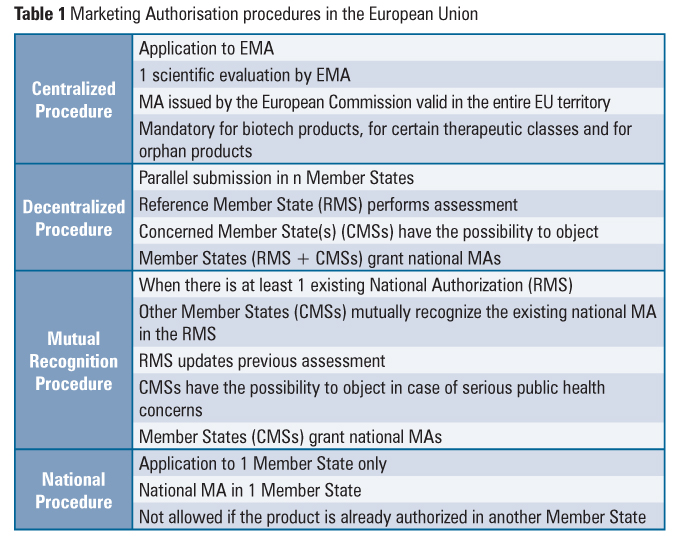
 Volume 2a Procedures For Marketing Authorisation Chapter 1
Volume 2a Procedures For Marketing Authorisation Chapter 1
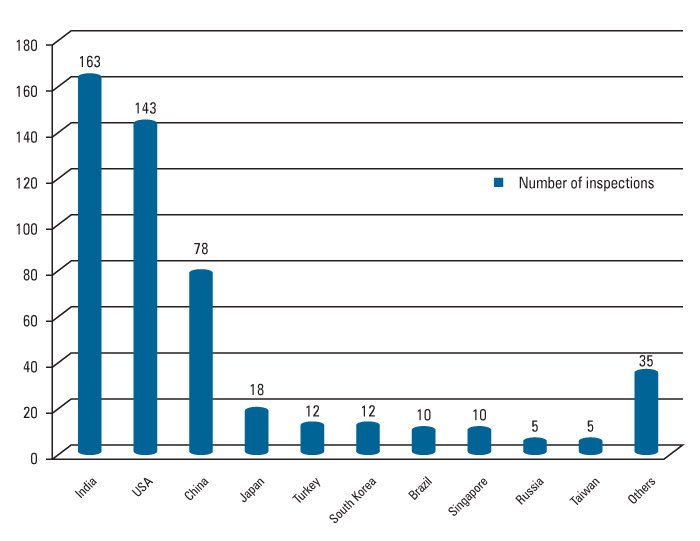
 Ema Cites Micro Labs Plant In India Fiercepharma
Ema Cites Micro Labs Plant In India Fiercepharma
 European Medicines Agency Loses Battle To End Uk Lease Over
European Medicines Agency Loses Battle To End Uk Lease Over
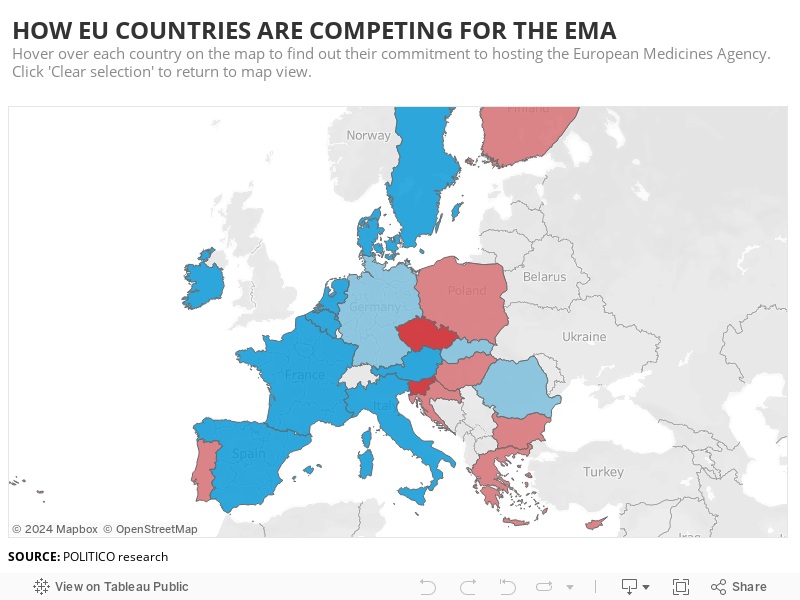 Hosting The Ema Who S Dead Serious Who S Along For The
Hosting The Ema Who S Dead Serious Who S Along For The
 Effectiveness Safety And Costs Of Orphan Drugs An Evidence
Effectiveness Safety And Costs Of Orphan Drugs An Evidence
Regulatory Newsletter N 22 April June 2018
 Gmp Oversight Of Medicines Manufacturers In The European Union
Gmp Oversight Of Medicines Manufacturers In The European Union
 Characteristics Of Non Randomised Studies Using Comparisons
Characteristics Of Non Randomised Studies Using Comparisons
 Gmp Logfile Lead Article Gmp Verlag Logfile Feature 28
Gmp Logfile Lead Article Gmp Verlag Logfile Feature 28
 Prac Considers That Benefit Risk Balance Of Tredaptive
Prac Considers That Benefit Risk Balance Of Tredaptive
 Pdf Comparison Of The Ema And Fda Guidelines On Ulcerative
Pdf Comparison Of The Ema And Fda Guidelines On Ulcerative
Technology Forecast Advanced Therapies In Late Clinical
 Brexit S Side Effects For Life Saving Medicines Europe
Brexit S Side Effects For Life Saving Medicines Europe
Eu Agency Faces 400m London Rent Bill After Post Brexit
 Regulatory Review Of Novel Therapeutics Comparison Of
Regulatory Review Of Novel Therapeutics Comparison Of
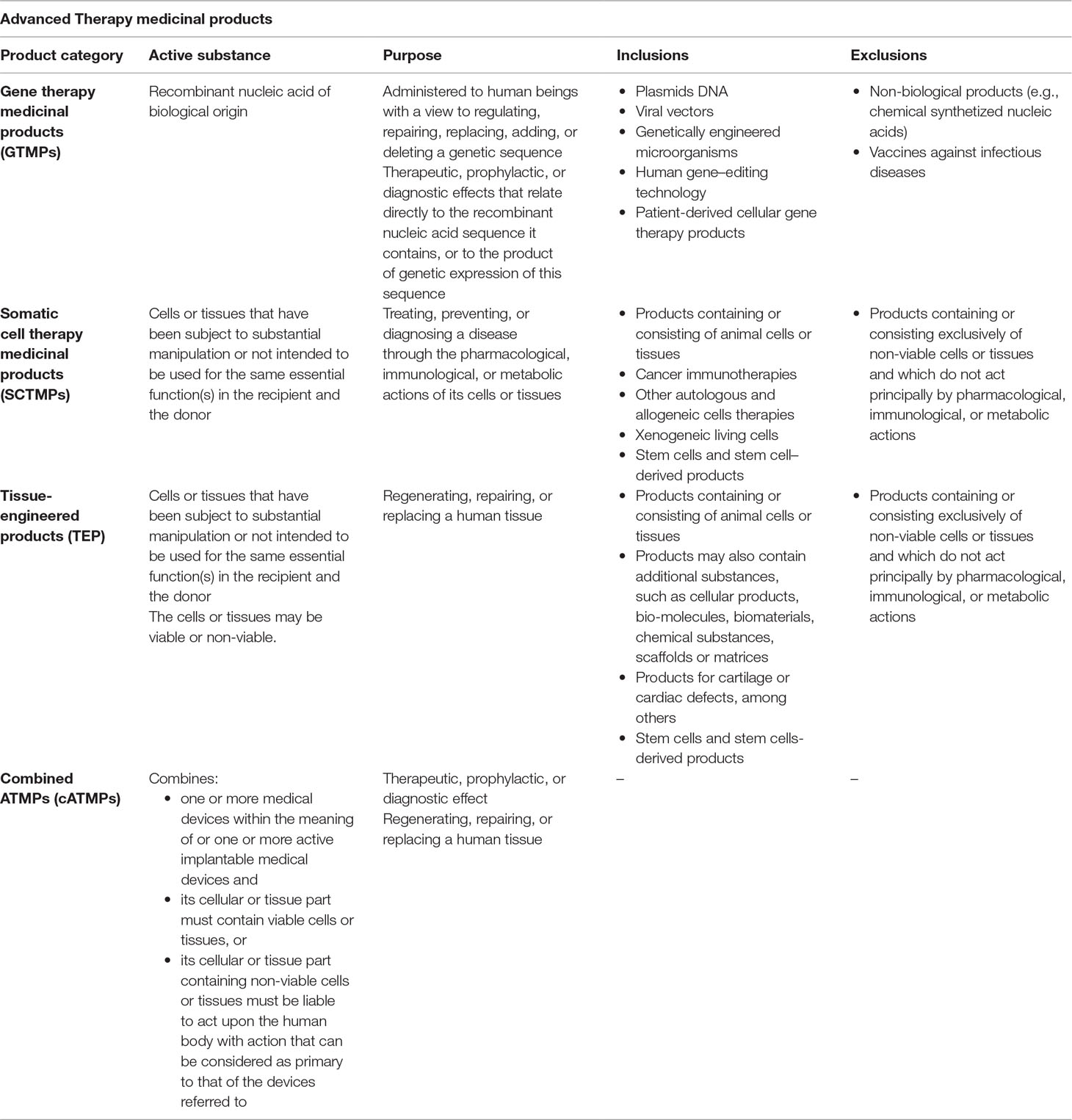 Frontiers Regulatory Framework For Advanced Therapy
Frontiers Regulatory Framework For Advanced Therapy
 The Funding You Are Entitled To Is Dependent On The Level
The Funding You Are Entitled To Is Dependent On The Level
 Regulatory Approval Of Pharmaceuticals Without A Randomised
Regulatory Approval Of Pharmaceuticals Without A Randomised
Ema Guidance Based On Hard Brexit Impact For Medical Devices
 Promoting And Protecting Public Health How The European
Promoting And Protecting Public Health How The European
 How The U S Compares To Europe On Biosimilar Approvals And
How The U S Compares To Europe On Biosimilar Approvals And
 Hosting The Ema Who S Dead Serious Who S Along For The
Hosting The Ema Who S Dead Serious Who S Along For The
 Similarities And Differences In The Oncology Drug Approval
Similarities And Differences In The Oncology Drug Approval
 Cancel A Medicine S Marketing Authorisation Or Other Licence
Cancel A Medicine S Marketing Authorisation Or Other Licence
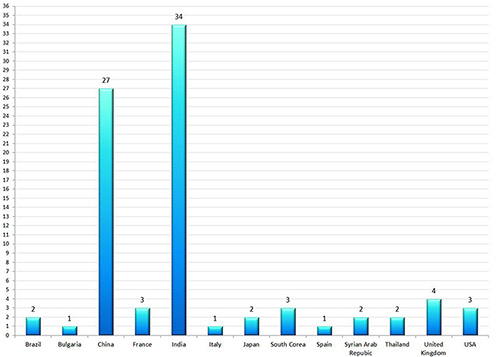 85 Company Listed With Gmp Non Compliance Statements In The
85 Company Listed With Gmp Non Compliance Statements In The
 Pdf Priority Review Drugs Approved By The Fda And The Ema
Pdf Priority Review Drugs Approved By The Fda And The Ema
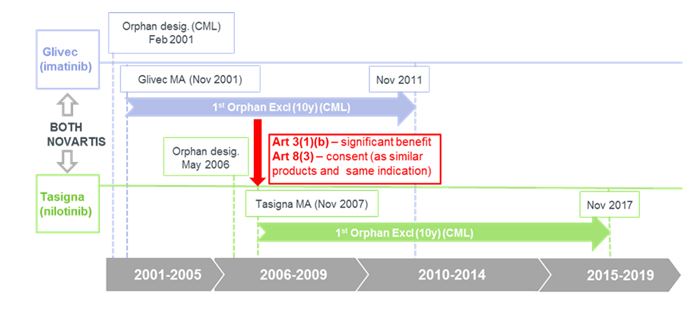
Post Marketing Withdrawal Of Analgesic Medications Because
 Brexit Uk Faces 520m Bill For Moving The European
Brexit Uk Faces 520m Bill For Moving The European
 Ema Follows Fda In Putting Pfizer S Xeljanz Under The
Ema Follows Fda In Putting Pfizer S Xeljanz Under The
 Navigating Ha Websites Locating Regulatory Information Dia
Navigating Ha Websites Locating Regulatory Information Dia
 Drugs And Devices Comparison Of European And U S Approval
Drugs And Devices Comparison Of European And U S Approval
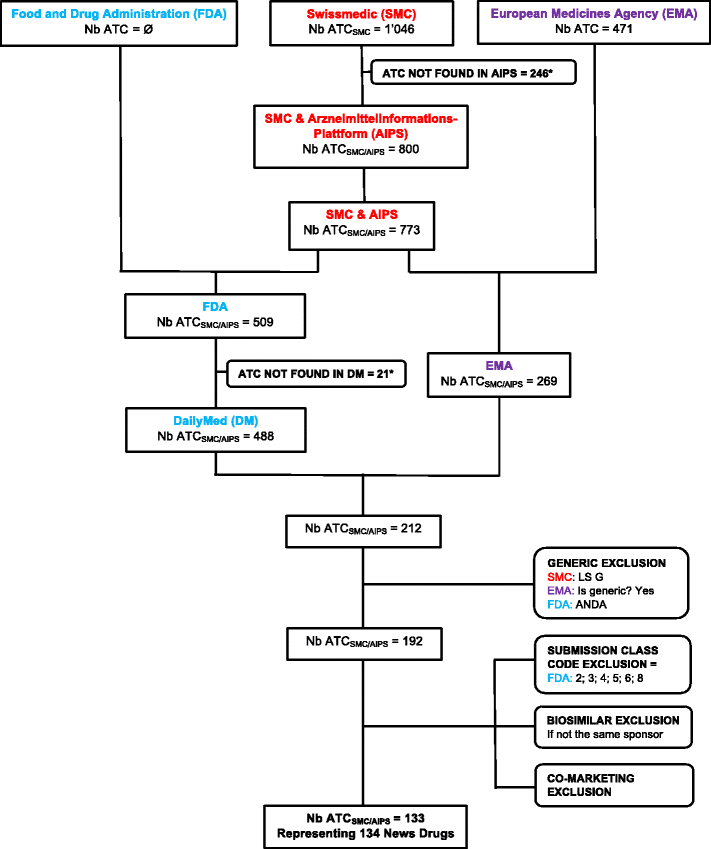 A Comparison Of New Drugs Approved By The Fda The Ema And
A Comparison Of New Drugs Approved By The Fda The Ema And
 Ex 99 2 3 A18 17578 1ex99d2 Htm Ex 99 2 Exhibit 99 2
Ex 99 2 3 A18 17578 1ex99d2 Htm Ex 99 2 Exhibit 99 2
 Hosting The Ema Who S Dead Serious Who S Along For The
Hosting The Ema Who S Dead Serious Who S Along For The
 Planning To Settle Abroad Here S How You Can Withdraw Your
Planning To Settle Abroad Here S How You Can Withdraw Your
 Harmonised Technical Guidance For Ectd Submissions In The Eu
Harmonised Technical Guidance For Ectd Submissions In The Eu
 The Funding You Are Entitled To Is Dependent On The Level
The Funding You Are Entitled To Is Dependent On The Level
Antimicrobial Resistance Ema Ameg Categorisation In
 How The U S Compares To Europe On Biosimilar Approvals And
How The U S Compares To Europe On Biosimilar Approvals And
 One Of Two Pfizer Adalimumab Biosimilar Applications
One Of Two Pfizer Adalimumab Biosimilar Applications
 Investigation Assessing The Publicly Available Evidence
Investigation Assessing The Publicly Available Evidence
 Ema Should Explain Why Oil Price Drops More Than 35 But
Ema Should Explain Why Oil Price Drops More Than 35 But
 Eiuc Eu Un Fellowship Programme 2018 Global Campus Of
Eiuc Eu Un Fellowship Programme 2018 Global Campus Of
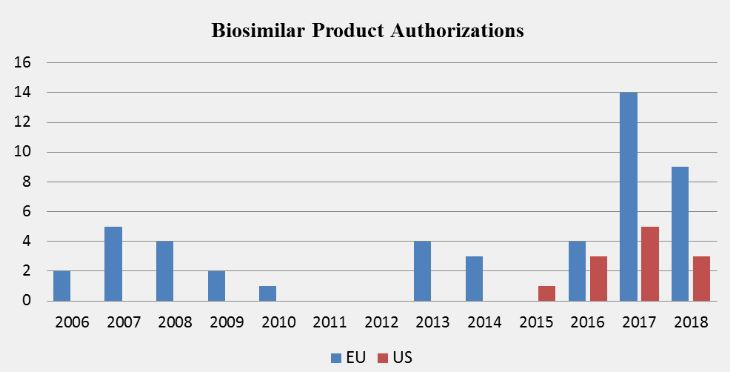
 Ema Officially Welcomed To New Premises In Amsterdam I
Ema Officially Welcomed To New Premises In Amsterdam I
 Pdf Global Regulatory Landscape Of Biosimilars Emerging
Pdf Global Regulatory Landscape Of Biosimilars Emerging
Public Pack Agenda Document For External Affairs And
 Similarities And Differences In The Oncology Drug Approval
Similarities And Differences In The Oncology Drug Approval









Post a Comment
Post a Comment